
 |
| Parameter |
Min |
Max |
Remarks |
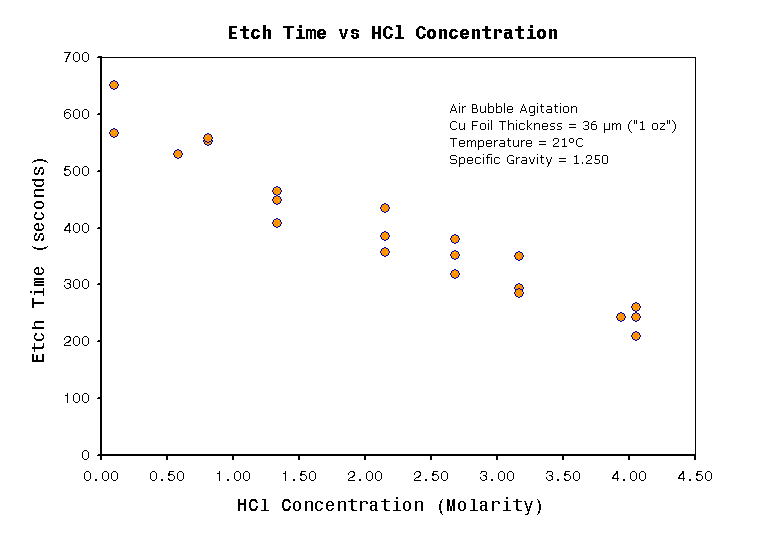
| Free acid
concentration (molarity) |
1.0 |
3.5 |
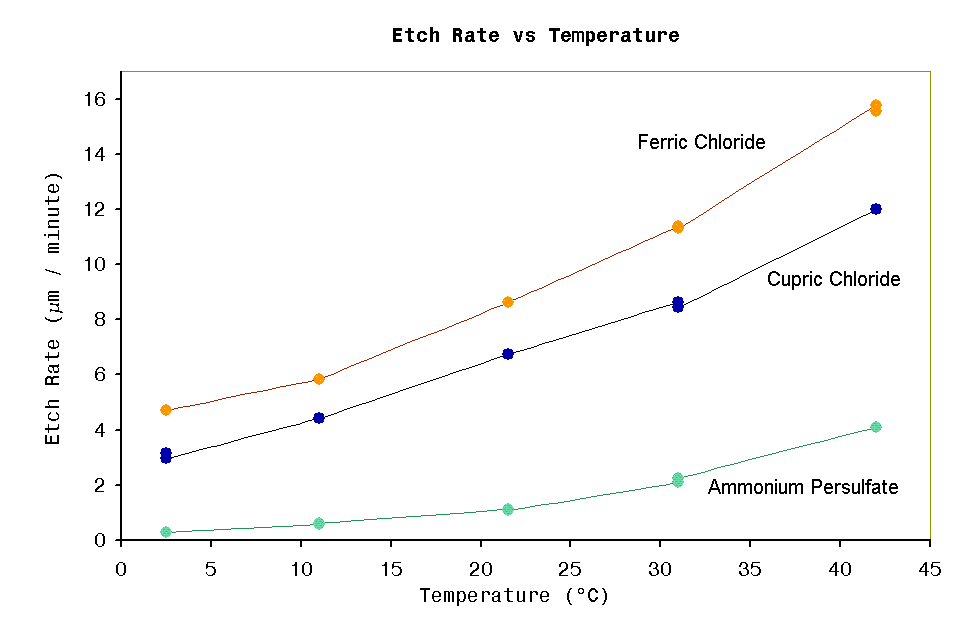
HCl fuming
increases Etch speed increases. (see note 1) |
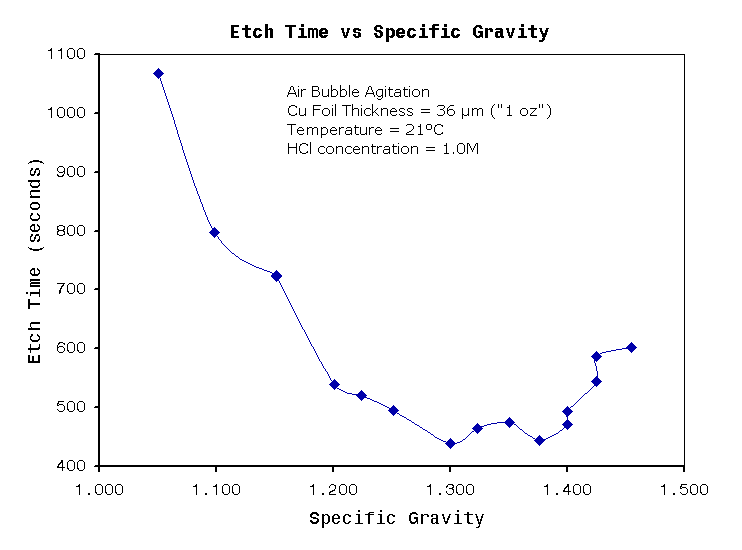
| Specific
gravity (g/cm3) |
1.220 |
1.380 |
Relatively
stable etch rate across range |
| Temperature
(°C) |
0 |
40 |
HCl fuming
increases. Etch speed increases. (see note 1) |
| Minimum bulk tank volume per board area (liters/cm2 ) |
0.016 |
- |
Assuming 50%
track coverage, double sided, 36 μm ("1oz") copper PCB. |
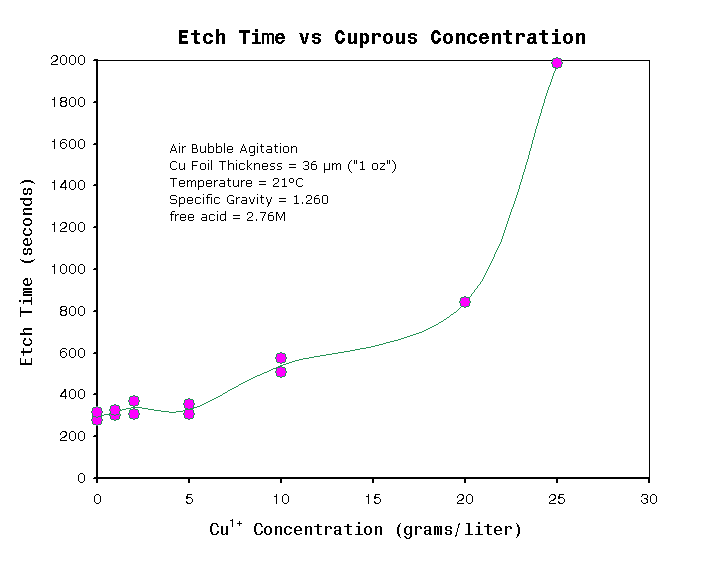
| Maximum monovalent copper ion, Cu1+ (g/liter) | - |
5 |
Color of
drops on white surface should appear bright green to olive dark green. Any signs of brown color indicates too high Cu1+. Also see Figure 5 |

| Method |
Chemical Ingredients | pros |
cons |
| Metal + Air |
Concentrated HCl Copper Metal Hydrogen Peroxide 3% (optional) |
Low cost Ingredients widely available |
Long time to prepare (3 to 10
days) |
| Copper(II) Oxides |
Concentrated HCl Copper Hydroxide or Copper(II) Oxide |
Quickest. Least amount of labor |
May prove difficult or costly
obtaining copper salts |
| molarity |
% weight |
grams/liter |
| 7.57 |
24.63 |
275.9 |
| 8.24 |
26.6 |
300.6 |
| 8.93 |
28.56 |
325.6 |
| 9.63 |
30.54 |
351.2 |
| 10.4 |
32.54 |
377.5 |
| 11.1 |
34.5 |
404.0 |
| 11.8 |
36.58 |
431.6 |
See also:
Questions:
Hey very nice article! thanks for that. I just have a question, is it possible to etch other alloys of copper like brass with Cupric Chloride? Thanks for your answer :-)