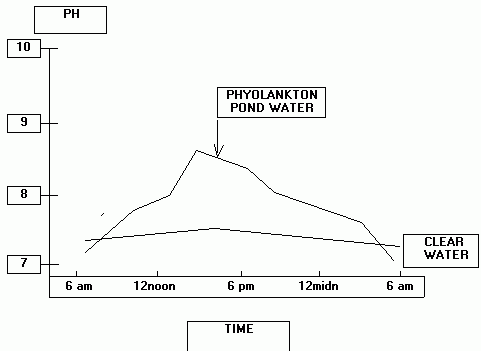
Tilapia can tolerate a wide range of pH. Direct pH toxicosis would be unusual for cultured tilapia. However, pH interacts with other water chemistry variables and strongly influences the toxicity to aquatic animals of ammonia and hydrogen sulfide. In terms of disease, pH effects mediated through these interactions are much more likely to be encountered in culture systems that support abundant phytoplankton. In phytoplankton rich culture water the pH will change gradually on a diurnal cycle. During periods of increased sunlight, carbon dioxide is reduced through uptake by plants for photosynthesis. As CO2 level declines, pH of the water increases. At night, photosynthesis is replaced by oxidative metabolism, CO2 is released into the water and pH value declines. The amplitude of the diurnal fluctuation is mediated by the density of phytoplankton, availability of sunlight and the buffering capacity of the water (as indicated by the alkalinity). Thus, source water of low alkalinity can have a profound effect on pH of the culture water. A stylized representation is given below of the diurnal pH fluctuation in pond water at two phytoplankton densities.

To visualize the extent of pH change, several pH measurements need to be taken through the day and night (e.g. 6:00 am [or just before sunrise], 12:00 noon, 6:00 pm and 12:00 midnight). If little to no phytoplankton are present in the culture water, the amplitude of this pH change will be minimal.
The hydrogen ion concentration (pH) of water exerts a major influence on the dissociation of ammonia in water to favor an increase in the concentration of the toxic NH3 relative to the less toxic, ionized NH4+. As the hydrogen ion concentration decreases and pH rises, more ammonia is shifted into the unionized toxic form. This relationship is discussed in greater detail in the section on ammonia.
Hydrogen sulfide also occurs in water in a dissociation equilibrium. An increased hydrogen ion concentration (decreased pH) causes an increase in the toxic form of hydrogen sulfide. Thus, H2S toxicity proceeds when pH is near neutral or lower. This relationship is explained in more detail in the section on hydrogen sulfide.