With nothing more than a few pieces of plumbing and a source of compressed air, you can build a remarkably simple device for attaining moderately low temperatures. It separates high-energy molecules from those of low energy. George O. Smith, an engineer of Rumson, N. I., discusses its theory and construction
The 19th century British physicist James Clerk Maxwell made many deep contributions to physics, and among the most significant was his law of random distribution. Considering. the case of a closed box containing a gas, Maxwell started off by saying that the temperature of the gas was due to the motion of the individual gas molecules within the box. But since the box was standing still, it stood to reason that the summation of the velocity and direction of the individual gas molecules must come to zero.
In essence Maxwell's law of random distribution says that for every gas molecule headed east at 20 miles per hour, there must be another headed west at the same speed. Furthermore, if the heat of the gas indicates that the average velocity of the molecules is 20 miles per hour, the number of molecules moving slower than this speed must be equaled by the number of molecules moving faster.
After a serious analysis of the consequences of his law, Maxwell permitted himself a touch of humor. He suggested that there was a statistical probability that; at some time in the future, all the molecules in a box of gas or a glass of hot water might be moving in the same direction. This would cause the water to rise out of the glass. Next Maxwell suggested that a system of drawing both hot and cold water out of a single pipe might be devised if we could capture a small demon and train him to open and close a tiny valve. The demon would open the valve only when a fast molecule approached it, and close the valve against slow molecules. The water coming out of the valve would thus be hot. To produce a stream of cold water the demon would open the valve only for slow molecules.
Maxwell's demon would circumvent the law of thermodynamics which says in essence: "You can't get something for nothing." That is to say, one cannot separate cold water from hot without doing work. Thus when physicists heard that the Germans had developed a device which could achieve low temperatures by utilizing Maxwell's demon, they were intrigued, though obviously skeptical. One physicist investigated the matter at first hand for the U. S. Navy. He discovered that the device was most ingenious, though not quite as miraculous as had been rumored.
It consists of a T-shaped assembly of pipe joined by a novel fitting, as depicted in Figure 234. when compressed air is admitted to the "leg" of the T, hot air comes out of one arm of the T and cold air out of the other arm! Obviously, however, work must be done to compress the air.
The origin of the device is obscure. The principle is said to have been discovered by a Frenchman who left some early experimental models in the path of the German Army when France was occupied. These were turned over to a German physicist named Rudolf Hilsch, who was working on low temperature refrigerating devices for the German war effort. Hilsch made some improvements on the Frenchman's design, but found that it was no more efficient than conventional methods of refrigeration in achieving fairly low temperatures. Subsequently the device became known as the Hilsch tube.
The Hilsch tube may be constructed from a pair of modified nuts and associated parts as shown in Figure 235. The horizontal arm of the T-shaped fitting contains a specially machined piece, the outside of which fits inside the arm. The inside of the piece, however, has a cross section which is spiral with respect to the outside. In the "step" of the spiral is a small opening which is connected to the leg of the T Thus air admitted to the leg comes out of the opening and spins around the one-turn spiral. The "hot" pipe is about 14 inches long and has an inside diameter of half an inch. The far end of this pipe is fitted with a stopcock which can be used to control the pressure in the system [see Fig. 236]. The "cold" pipe is about four inches long and also has an inside diameter of half an inch. The end of the pipe which butts up against the spiral piece is fitted with a washer, the central hole of which is about a quarter of an inch in diameter. Washers with larger or smaller holes can also be inserted to adjust the system.
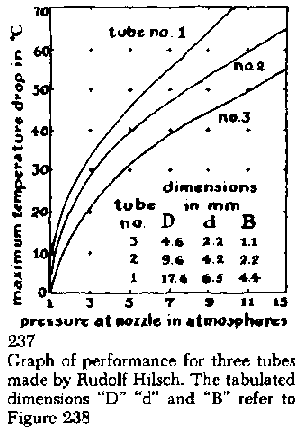
Three factors determine the performance of the Hilsch tube; the setting of the stopcock, the pressure at which air is admitted to the nozzle, and the size of the hole in the washer. For each value of air pressure and washer opening there is a setting of the stopcock which results in a maximum difference in the temperature of the hot and cold pipes [see Fig. 237]. When the device is properly adjusted, the hot pipe will deliver air at about 100 degrees Fahrenheit and the cold pipe air at about -70 degrees (a temperature substantially below the freezing point of mercury and approaching that of "dry ice"). When the tube is adjusted for maximum temperature on the hot side, air is delivered at about 350 degrees F. It must be mentioned, however, that few amateurs have succeeded in achieving these performance extremes. Most report minimums on the order of -10 degrees and maximums of about + 140 on the first try. Despite its impressive performance, the efficiency of the Hilsch tube leaves much to be desired. Indeed, there is still disagreement as to how it works. According to one explanation, the compressed air shoots around the spiral and forms a high-velocity vortex of air. Molecules of air at the outside of the vortex are slowed by friction with the wall of the spiral. Because these slow-moving molecules are subject to the rules of centrifugal force, they tend to fall toward the center of the vortex. The fast-moving molecules just inside the outer layer of the vortex transfer some of their energy to this layer by bombarding some of its slow-moving molecules and speeding them up. The net result of this process is the accumulation of slow-moving, low-energy molecules in the center of the whirling mass, and of high-energy, fast-moving molecules around the outside. In the thermodynamics of gases the terms "high energy" and "high velocity" mean "high temperature." So the vortex consists of a core of cold air surrounded by a rim of hot air.
The difference between the temperature of the core and that of the rim is increased by a secondary effect which takes advantage of the fact that the temperature of a given quantity of gas at a given level of thermal energy is higher when the gas is confined in a small space than in a large one; accordingly when gas is allowed to expand, its temperature drops. In the case of the Hilsch tube the action of centrifugal force compresses the hot rim of gas into a compact mass which can escape only by flowing along the inner wall of the "hot" pipe in a compressed state, because its flow into the cold tube is blocked by the rim of the washer.
The amount of the compression is determined by the adjustment of the stopcock at the end of the hot pipe. In contrast, the relatively cold inner core of the vortex, which is also considerably above atmospheric pressure, flows through the hole in the washer and drops to still lower temperature as it expands to atmospheric pressure obtaining inside the cold pipe.
Apparently the inefficiency of the Hilsch tube as a refrigerating device has barred its commercial application. Nonetheless amateurs who would like to have a means of attaining relatively low temperatures, and who do not have access to a supply of dry ice, may find the tube useful. when properly made it will deliver a blast of air 20 times colder than air which has been chilled by permitting it simply to expand through a Venturi tube from a high-pressure source. Thus the Hilsch tube could be used to quick- freeze tissues for microscopy, or to chill photomultiplier tubes. But quite apart from the tube's potential application, what could be more fun than to trap Maxwell's demon and make him explain in detail how he manages to blow hot and cold at the same time?
Incidentally, this is not a project for the person who goes in for commercially made apparatus. So far as I can discover Hilsch tubes are not to be found on the market. You must make your own. Nor is it a project for the experimenter who makes a speciality of building apparatus from detailed specifications and drawings. The dimensions shown in the accompanying figures are only approximate. Certainly they are not optimum values. But if you enjoy exploration, the device poses many questions. What would be the effect, for example, of substituting a divergent nozzle for the straight one used by Hilsch? Why not create the vortex by impeller vanes, such as those employed in the stator of turbines? Would a spiral chamber in the shape of a torus improve the efficiency? What ratio should the diameter of the pipes bear to the vortex chamber and to each other? Why not make the spiral of plastic, or even plastic wood? One can also imagine a spiral bent of a strip of brass and soldered into a conventional pipe coupling. Doubtless other and far more clever alternatives will occur to the dyed-in-the-wool tinkerer.